Disclaimer: This page is intended for healthcare professional only.
The Effect of Enzyme Supplementation on Symptoms and Duodenal Histology in Celiac Patients1
This double-blind, randomised clinical trial was carried out using GluteGuard® compared with placebo. Twenty patients were randomized to receive a placebo (n=6) or a GluteGuard® (n=14) tablet given daily. Both groups were challenged with 1g of gluten daily and patients recorded and graded their symptoms over a period of 42 days. Small bowel histology was monitored at the start and end of the study.
Patients who received GluteGuard® over 1-14 days were significantly less likely to report symptoms than the placebo group.

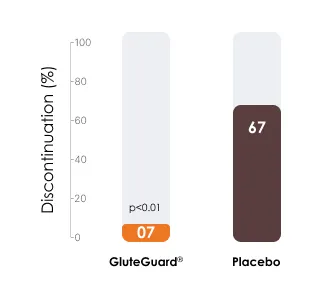
Significantly fewer patients discontinued the gluten challenge in the GluteGuard® group vs. those who received placebo.
41.7% of patients taking GluteGuard® reported that they had no symptoms at the end of the trial, whilst 58.3% reported only mild symptoms.
67% patients in the placebo group and 7% in the treatment group withdrew in the first 14 days due to the severity of their symptoms from the gluten challenge.
No detrimental changes in small bowel histology for the treatment group, despite 42 days of gluten challenge.
No adverse effects from GluteGuard® were recorded during the trial.
The trial showed that oral enzyme therapy with GluteGuard® was effective in preventing the symptoms induced by gluten challenge, with a statistically significant difference between treatment and placebo after 14 days. Patient-assessed well-being scores on Day 14 were statistically improved with Gluteguard (Gluteguard: 8.4 vs. placebo: 6.1; p =< 0.01)
This product is not intended to treat or prevent celiac disease.
The only treatment for celiac disease is a strict, lifelong adherence to a gluten-free diet. GluteGuard® was demonstrated to protect celiac patients against a robust gluten challenge that resembled ongoing exposure to gluten, often in the form of inadvertent or accidental gluten ingestion. GluteGuard® is intended as an adjunct to the gluten free diet.
The Effect of Enzyme Therapy on Skin Symptoms and Immune Responses in Patients with Dermatitis Herpetiformis2
1.Cornell, H.J., Czyzewska, A., Macrae, F.A., Rydzewska, G., Nasierowska-Gutmejer, A., Bednarczuk, A., & Stelmasiak, T. (2016). The effect of enzyme supplementation on symptoms and duodenal histology in celiac patients. International Journal of Celiac Disease, 4, 40-47.
GluteGuard® is indicated to protect against the symptoms of accidental gluten ingestion. Consult the warnings, precautions, and user instructions for information to help you assess the risks and benefits. This information is also available by contacting us at 1-877-595-2364. Always direct the patient to read the label.
